(通讯员 刘文叶)近日,jxf吉祥官网总站鱼类逆境发育遗传学团队通过研究,发现了Cu2+通过表观调控影响斑马鱼肌原纤维分化机制。相关研究论文以 “Copper ions impair zebrafish skeletal myofibrillogenesis via epigenetic regulation”为题,在线发表于《The FASEB Journal》。
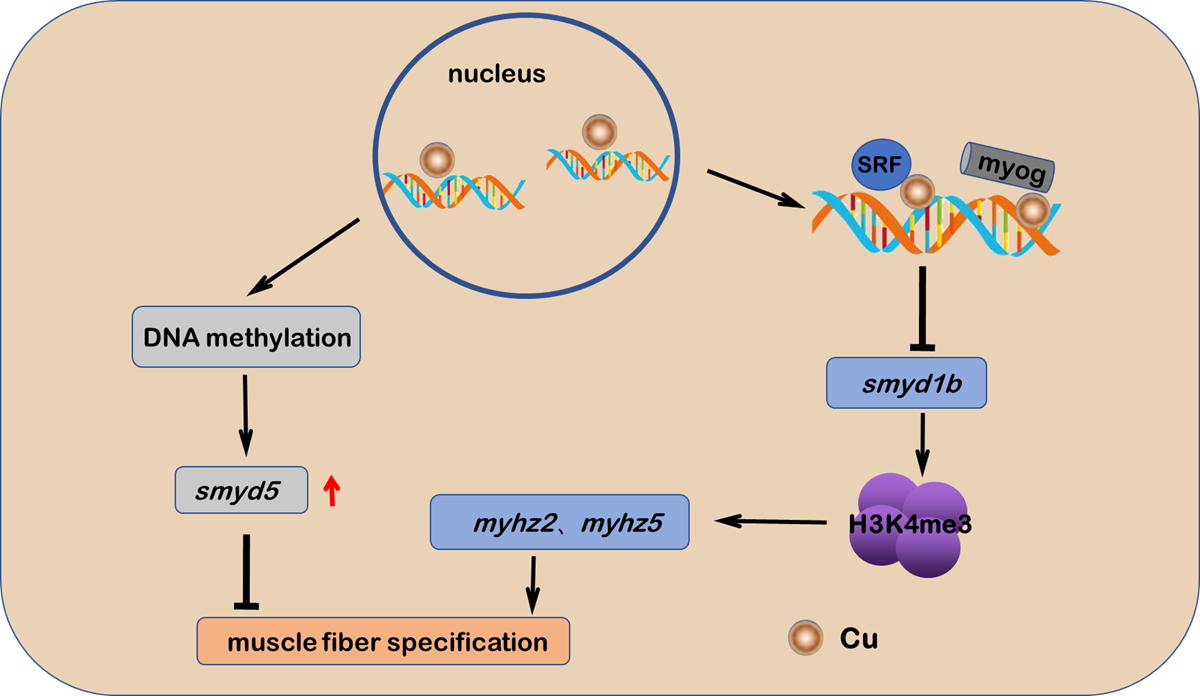
Cu2+调控斑马鱼肌原纤维分化的模式图
铜作为一种生物体必需的微量元素,会参与一系列的生理过程,而铜在生物体内的稳态失衡也会导致机体发育异常和疾病发生。研究铜稳态代谢失衡下的鱼类胚胎发育学对研究鱼类环境胁迫的生物学响应以及适应性进化具有重要意义。该团队以斑马鱼为模型,发现Cu2+胁迫可通过抑制Srfa和Myog转录活性下调smyd1b的表达。另外,Cu2+胁迫通过下调由smyd1b调控的甲基化组蛋白H3K4me3的表达以及改变smyd5启动子甲基化水平来抑制斑马鱼肌原纤维的分化。
硕士研究生靳晓东和博士研究生刘文叶为该论文共同第一作者,刘静霞教授为该论文的通讯作者。该研究得到了“国家自然科学基金”(32070807)、"蓝色粮仓科技创新"重点专项(2018YFD0900101)、中央高校基本科研业务费华中农业大学交叉专项(2662018JC024)等项目的资助。
近年来,本团队围绕铜稳态代谢失衡下的鱼类胚胎发育学,解析了环境铜过载或铜转运基因突变等引起的鱼类胚胎细胞中的铜稳态代谢失衡进而导致的神经系统、造血系统等发育缺陷,研究成果已相继发表于BBA-GRM,Cell Communication Signaling,Frontiers in Immunology等杂志。
审核人:刘静霞
【英文摘要】
Unbalanced copper (Cu2+) homeostasis is associated with the developmental defects of vertebrate myogenesis, but the underlying molecular mechanisms remain elusive. In this study, it was found that Cu2+ stressed zebrafish embryos and larvae showed reduced locomotor speed as well as loose and decreased myofibrils in skeletal muscle, coupled with the downregulated expression of muscle fiber markers mylpfa and smyhc1l and the irregular arrangement of myofibril and sarcomere. Meanwhile, the Cu2+ stressed zebrafish embryos and larvae also showed significant reduction in the expression of H3K4 methyltransferase smyd1b transcripts and H3K4me3 protein as well as in the binding enrichment of H3K4me3 on gene mylpfa promoter in skeletal muscle cells, suggesting that smyd1b—H3K4me3 axis mediates the Cu2+-induced myofibrils specification defects. Additionally, whole genome DNA methylation sequencing unveiled that the gene smyd5 exhibited significant promoter hyper-methylation and increased expression in Cu2+ stressed embryos, and the ectopic expression of smyd5 in zebrafish embryos also induced the myofibrils specification defects as those observed in Cu2+ stressed embryos. Moreover, Cu2+ was shown to suppress myofibrils specification and smyd1b promoter transcriptional activity directly independent of the integral function of copper transporter cox17 and atp7b. All these data may shed light on the linkage of unbalanced copper homeostasis with specific gene promoter methylation and epigenetic histone protein modification as well as the resultant signaling transduction and the myofibrillogenesis defects.
论文链接:https://faseb.onlinelibrary.wiley.com/doi/10.1096/fj.202100183R